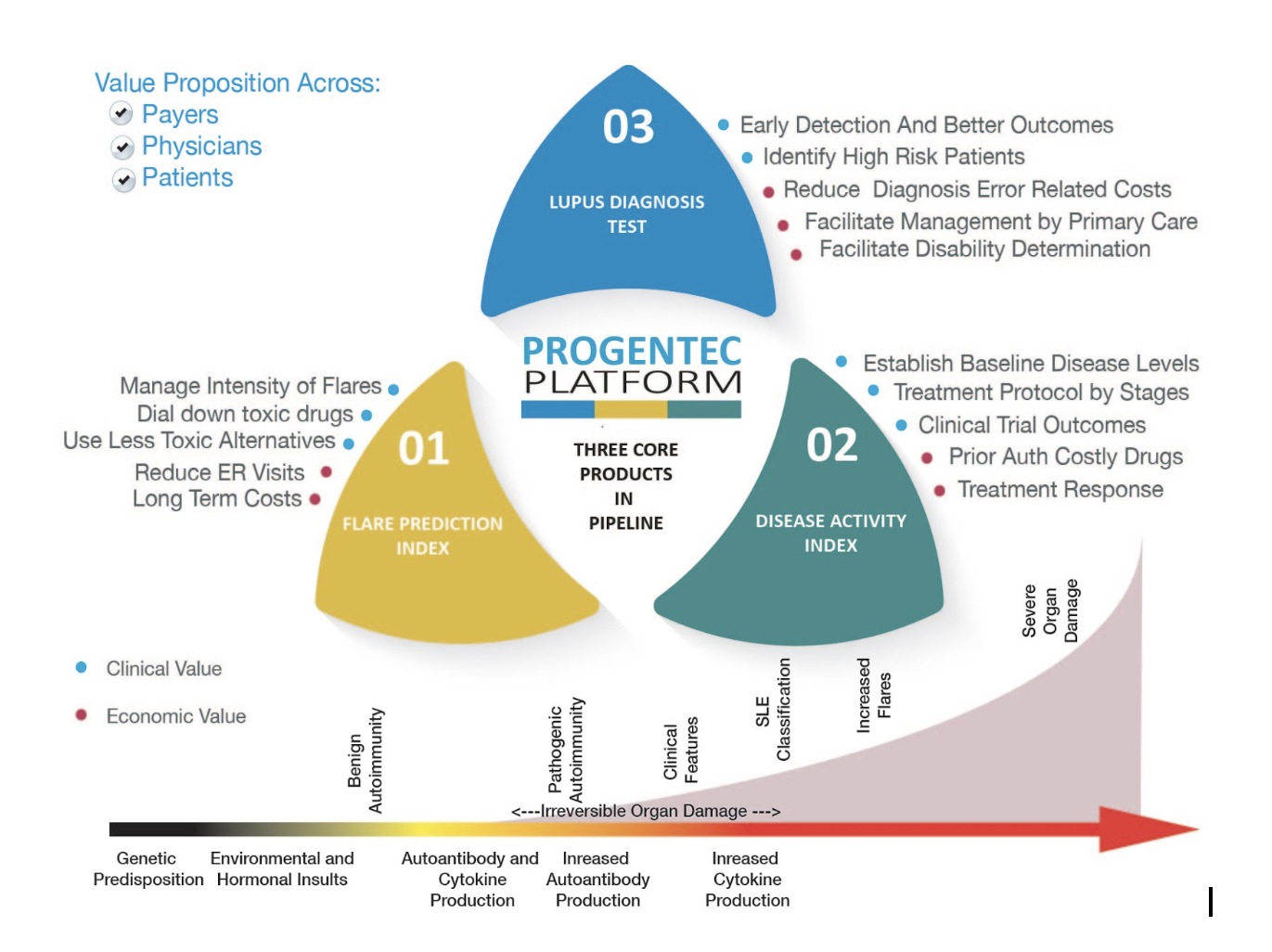
The company has identified three tests that can address unmet medical needs and with significant commercial potential in the near future.
The schematic below provides an overview of these tests:
aiSLE DX - Flare Prediction Index
Beta launch: Mid 2020
The Flare Prediction Index test is the most advanced in terms of test development. It involves the development of a Soluble Mediator Score that can accurately predict flares 6-8 weeks prior to an actual flare occurrence. Some of the salient features of this test are:
Lupus is emerging as a major therapeutic area of focus as there has been very little innovation in the pharmaceutical world for Lupus. It is a disease with very high mortality and morbidity leading to significant medical and economic costs.
No tests exist today to accurately predict Lupus Flares and therefore, treatment options are limited to use of steroids and other toxic medicines.
Flares are expensive for Payers and the Healthcare system - patients experience about 1.7 flares on an average each year at a cost of $12K per flare.
Long term organ damage and its resultant costs are high - A Flare Prediction test can help manage the frequency and intensity of Flares.
Advance information of an impending Flare can help physicians dial down toxic drugs and resort to use of less toxic drugs that can be used ahead of the flare.
aiSLE DX - Disease Activity Index
Launch in 2020
Our second test, development for which is progressing closely on the heels of the first test, is aimed at providing a reliable measure of the underlying disease activity. Unlike the first test which aims at predicting a future disease activity, this test provides a measure of the concurrent disease activity. Some of the salient features are:
Currently, disease activity is measured using subjective scores such as SLEDAI. This is not a satisfactory metric and there is thus a high demand for a reliable set of metrics for better disease management practices.
Biologics for the management of Lupus (such as Benlysta with an Rx volume of about 100K each year as of 2017) are expected to grow significantly over time. In addition, new and expensive biologics are entering the market in the coming years. This creates a unique opportunity for the introduction of a reliable disease activity measure.
From a payer’s perspective, disease activity test could be a powerful tool for better drug utilization management - especially as more expensive biologics come to the market.
Many clinical trials fail to establish achievement of end points for want of better disease activity scores. Our Disease Activity Index test could be a valuable tool for use in better design of clinical trials. Additionally, the test can also help in recruitment of the right kind of patients based on their underlying baseline disease activity.
Tracking response to treatment in Lupus patients is another major unmet need in the market.
aiSLE DX - Lupus Diagnosis Index
Launch in 1H 2021
Our last test in the trio of lupus related tests, is the Lupus Diagnosis Test. This test is aimed at diagnosing lupus well before symptoms begin to show. Lupus is a difficult disease condition to diagnose and patients often go through multiple diagnoses before a conclusive diagnosis of Lupus is made. Usually, this happens when significant organ damage has already taken place.
The tests available in the market for diagnosis of lupus do not offer a high degree of sensitivity and specificity.
There is an increase in focus by payers on accurate diagnosis as a requirement for initiating treatment on newer and expensive treatments
Early testing and diagnosis can help manage patients and ensure better outcomes in both the short term as well as long term.
Progentec is also working on other applications of the platform technology to provide additional diagnostic tests to help manage lupus patients and their needs.